i.Any warnings, precautions or measures to be taken by the patient with regard to reciprocal interference with reasonably foreseeable external influences, medical examinations or environmental conditions
Warnings
- ·Please read the user manual carefully before use. Please pay special attention to all warnings and precautions to avoid accidents.
- ·Please select the indication patients carefully. The use of this product has the same risks as conventional intracranial stent implantation, including acute vascular occlusion; Acute and subacute thrombosis; Vascular dissection; Vascular bleeding and other complications.
- ·Patients who are allergic to Sirolimus, polylactic acid-glycolic acid (PLGA), polybutyl methacrylate (PBuMA) and Nitinol may experience an allergic reaction to this product.
- ·The intracranial self-expanding drug coated stent system is delivered sterile and pyrogen-free for one-time use only. Re-usable, re-processing or re-sterilization may result in structural deformation and failure of the product, and contamination may cause infection or cross-infection of the patient, which may result in injury, illness or death to the patient.
- ·The procedure must be performed by a qualified professional who has received professional training in intracranial vascular stenting.
- ·Please inspect the packaging thoroughly before use. If the carton packaging is opened or damaged, or if the aluminum foil packaging bag or Tyvek 1059B PET/PE composite film packaging bag is compromised, do not use the product.
- ·Before use, please pay attention to the expiration date of the product, do not use expired products.
- ·This product should not be in contact with organic solvents. Do not immerse the product in normal saline for a long time.
- ·This product requires the use of anticoagulation and/or antiplatelet therapy. This product should not be used in patients who are not likely to comply with the recommended antiplatelet regimen.
Precautions
- After the stent is implanted, you will rest in a hospital unit where nurses and doctors can monitor you closely as you begin to recover.
- You may be asked to stay in bed for several hours and have some bruising and soreness at the area where the catheter was inserted, which is normal. If you received a sedative, you may feel sleepy or forgetful. Gradually, you will begin to feel normal. Pressure may be applied to the area of the incision to promote healing and prevent bleeding. It may be one or more days before you are discharged from the hospital.
- ·Follow the recommendations of your physician.
- ·Return to normal activities gradually and ask your doctor about specific exercise or strenuous activities.
- ·Let your doctor know about any changes in lifestyle you make during your recovery period.
- ·Keep up with all follow-up appointments, including any laboratory blood tests.
- ·Carry your Patient Implant Card at all times and show it to any medical professional who treats you, e.g. for dental work, medical care or when reporting to an emergency center.
- ·Register the stent and the conditions under which it can be scanned safely with the Medic Alert Foundation (www.medicalert. org) or equivalent organization.
ii.Expected lifetime of the device and any necessary follow up
- The lifetime of Cometiu stent is 10 years according to the performance Verification. After the stent is implanted, the stent remains permanently in the artery.
- Follow- Up Examinations: You will need to see the doctor who implanted your stent for routine follow-up examinations, including laboratory blood testing.
iii. Any other information to ensure safe use of the device by the patient
Medications
Follow your doctor’s instructions exactly regarding the use and dosage of medications prescribed. Report side effects from medications to your doctor immediately. These may include headaches, nausea, vomiting or rash.
Magnetic Resonance Imaging (MRI)
- If you require a magnetic resonance imaging (MRI) scan, tell your doctor or MRI technician that you have a Cometiu and present your patient implant card.
- In non-clinical testing, the Cometiu stent has been shown to be MR Conditional for single stents up to 40 mm in length. A patient with this device can be safely scanned in an MR system that meets the following conditions:
- ·Static magnetic field of 3.0T or less.
- ·Maximum spatial field gradient of 6.808 T/m or less.
- ·For radiofrequency heating of MRI, whole body averaged specific absorption rate (SAR) of ≤ 2.4 W/kg for 1.5 T and 3.0 T MRI system in 15 minutes, and the maximum temperature rise of a single stent did not exceed 1.5°C.The effect of MRl related heating for overlapping stents or stents with fractured struts is unknown.
- ·The static magnetic field conditions do not cause stent displacement.
- ·No results were shown in non-clinical tests to rule out the possibility of stent movement at static magnetic field strengths above 6.808T/m.
- ·Artifacts may have an effect if the imaging area is close to or at the stent implantation site.
Keep your ID Card Handy
Show your implant card if you report to an emergency room. This card identifies you as a patient who has had a stent implanted.
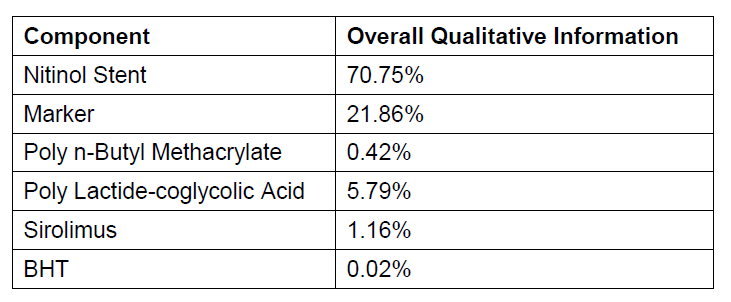
iv.Overall qualitative and quantitative information on the materials and substances to which patients can be exposed
Table 1 Qualitative and Quantitative Information on Implants